Honey I Need to Use the Microwave Now, and Can You Read Me the Recipe for a Good Wet Weather Golf Ball from the Back of that Box of Nike Golf Balls
Just wait, you may find those words singing from your lips in the future. So, what’s the inspiration for this post? A Nike patent application of course. Today a patent application published as US Pub. No.
[0022] The cover layer on the golf ball is formed of a semi-crystalline thermoplastic polymer material. A thermoplastic polymer material is a polymer that changes phase upon heating and cooling. For example, a thermoplastic polymer that is solid at room changes to a liquid upon being heated to a temperature above a certain temperature. In contrast, a thermoset polymer does not change phase upon heating, but instead merely degrades. Generally, thermoset materials include permanently bonded cross-linkages between the long chain polymer molecules, while thermoplastic materials include few if any permanently bonded cross-linkages.
.
.
.
[0025] In step 104 of process 100 shown in FIG. 1, the finished golf ball is provided to an end-user consumer. Generally, latter steps in method 100, steps 106, 108 and 110, are performed by a party other than the party responsible for manufacturing the golf ball. The golf ball thereby undergoes aftermarket customization. The customization may be performed by anyone post-manufacture, such as the end-user him or herself, or by (for example) a golf pro at a golf pro shop.
[0026] The next step in the customization process 100, as shown in FIG. 1, is step 106 of heating the golf to a preselected temperature. The preselected temperature may generally be any temperature that delivers sufficient heat energy to break at least some of the weak associations between the polymer molecules in the crystalline phase of the semi-crystalline thermoplastic polymer material. The exact value of this temperature will depend on the semi-crystalline thermoplastic polymer material used in the golf ball cover layer. This heating will therefore decrease the amount of crystalline phase and increase the amount of amorphous phase, decreasing the degree of crystallinity.
[0027] In particular embodiments, the preselected temperature may be any temperature that is higher than the glass transition temperature (“T.sub.g”). For example, the preselected temperature may be any temperature that is higher than the softening point of the polymer material. The softening point of a polymer material is the temperature at which a polymer material has softened to the point that a specific load indents the material to a predetermined degree. The softening point may be determined in accordance with the Vicat method, as detailed in ASTM D 1525 and ISO 306. The softening point is also a temperature at which a significant number of the crystalline domains disassociate into an amorphous phase, although the change from crystalline to amorphous phase takes place to some degree over a wide range of temperatures beginning with a lower bound of the glass transition temperature.
[0028] In other embodiments, the preselected temperature may be any temperature that is higher than the softening point and less than the melting point of the semi-crystalline thermoplastic polymer material. The melting point, T.sub.m, is generally the temperature at which the polymer changes entirely from the semi-crystalline state into a viscous flow state. In other words, the melting point is the temperature at which all crystalline domains are completely disassociated, such that the polymer is only in the amorphous phase. In the range between the softening point and the melting point, the preselected temperature is sufficiently high so as to dissociate most of the crystalline domains without turning the thermoplastic polymer material into a flowable liquid. This range therefore achieves reduction of the degree of crystallinity without making the thermoplastic material difficult to handle and process.
[0029] After step 106, a desired hardness of the golf ball is selected in step 108. Generally, the hardness of a semi-crystalline thermoplastic material is proportional to the degree of crystallinity. The crystalline domains in the polymer material are physically harder than the amorphous domains, due to the crystalline close packing structure. Therefore, a greater degree of crystallinity will result in a greater hardness of the polymer material as a whole. The desired hardness of the golf ball is selected by the end-user consumer golfer, or by another person such as a golf pro, so that the golf ball will achieve desired play characteristics. For example, if the golfer intends to play on a long course the golf may select a higher hardness as the desired hardness. Alternatively, if the golfer is playing in the cold, the golf may select a lower hardness as the desired hardness.
[0030] Next, the golf ball is cooled at a preselected rate that corresponds to the selected desired hardness in step 110. Generally, the preselected rate in step 110 controls the degree of crystallinity formed in the semi-crystalline thermoplastic polymer material as the material cools. As discussed above, the degree of crystallinity decreases during heating because the heat energy causes the weak associations holding the crystalline domains together to disassociate. However, during cooling the weak associations may bring portions of the polymer molecules back into a crystalline phase. However, the polymer molecules must be able to move and bend on the molecular level in order for the polymer molecules to property align into a crystalline phase. Therefore, slowly cooling the heated semi-crystalline thermoplastic material will leave sufficient heat energy in the material for the polymer molecules to move and so align into crystalline domains. However, quickly cooling the heated material will remove that heat energy before crystalline domains can be formed. Accordingly, the rate at which the semi-crystalline thermoplastic material cools controls the degree of crystallinity.
.
.
.
[0035] FIG. 3 shows an end-user performing the process as shown in FIGS. 1 and 2. Specifically, the end user 200 may receive a kit 202 containing at least one finished golf ball 204 in step 104. Kit 202 shown in FIG. 3 also includes a graphical representation of the relationship between various preselected cooling rates and resulting hardness of the golf ball, and a stand 206 for holding golf ball 204.
[0036] Next, in step 106, end-user 200 heats golf ball 204 to a preselected temperature using a microwave 208 by placing golf ball 204 on stand 206 inside microwave 208.
[0037] In some embodiments, the cover layer of golf ball 204 may include a microwave absorbing additive. Generally, a microwave absorbing additive turns microwaves into heat. The microwave absorbing additive in the cover layer therefore ensures that the cover layer is sufficiently heated when end-user 200 heats the golf ball using a microwave 208. Use of a microwave absorbing additive in the cover layer can advantageously avoid the need to microwave golf ball 204 for a long time period, which might damage other portions of the golf ball such as a rubber core. The microwave absorbing additive may be any known microwave absorbing additive, such as (for example) a glycerine or a carbon black.
[0038] Then the end-user chooses a desired hardness and selects one of the three cooling paths in step 110. If end user 200 desires a soft cover layer on the golf ball, then the end-user may perform step 120 by quenching golf ball 204 in a cold water bath 210. In a particular embodiment, the cold water bath may have a temperature of between about 4.degree. C. and about 13.degree. C., and golf ball 204 may be quenched for a time period of from about one minute to about five minutes. However, a variety of alternative methods of quickly cooling the golf ball are within the scope of step 120, as are known in the art of heat exchangers.
[0039] Alternatively, if end-user 200 desires a harder cover layer on golf ball 204, end-user 200 may perform steps 112 and 116 or step 114. In step 112, end-user 200 cools golf ball 204 in a warm water bath 212. The warm water bath 212 may have a temperature of between about 40.degree. C. and about 70.degree. C. Golf ball 204 may be placed in warm water bath 212 for a time period of from about one minute to about 10 minutes. Subsequently, after step 112, golf ball 204 may be removed from warm water bath 212 and placed on stand 206 to air cool to room temperature.
[0040] Thirdly, golf ball 204 may be allowed to slowly air cool, without an intermediate cooling step, as in step 114.
[0041] Generally, FIG. 3 shows an embodiment of how an end-user may perform the various steps in method 100 using readily available household equipment. However, each of steps 106, 120, 112 and 116, and 114 are intended to broadly encompass any heat exchange process that heats or cools the golf ball at the indicated rate so as to achieve the desired hardness. For example, the heating process may use a boiling water bath, or a hot air source such as a strong hair drier. The use of readily available household equipment makes the process easy for an end-user consumer to perform. However, larger scale heat exchange systems may be used in other embodiments, such as when the method is performed in a golf pro-shop.
.
.
.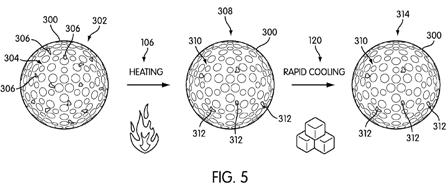
[0044] FIG. 5 shows a representative change in degree of crystallinity as the golf ball undergoes heating step 106 and rapid cooling step 120. Specifically, golf ball 300 starts out having a cover layer in state 302, which may be the state the golf ball was manufactured in. On the cover layer are crystalline domains 306 and amorphous domains 304. Although the crystalline domains 306 are shown in FIG. 5 as being visible areas, this representation is made merely to convey the concept of the change in degree of crystallinity. In fact, the crystalline domains are on the order of microns or even nanometers, are randomly dispersed throughout amorphous phase 304, and are not visible to the naked human eye. As shown in FIG. 5, first state 302 of golf ball 300 prior to heating may include a moderate degree of crystallinity, having a medium amount of crystalline domains.
[0045] Golf ball 300 then undergoes heating step 106. As discussed above, this heating reduces the degree of crystallinity by breaking up the weak associations holding the crystalline domains together. Therefore, only a few crystalline domains 312 remain when the golf ball is in state 308 after heating.
[0046] Golf ball 300 then undergoes rapid cooling step 120, as discussed above. Because the rapid cooling removes all of the heat energy, the polymer molecules cannot align themselves so as to form new crystalline domains. In other words, rapid cooling step 120 locks the degree of crystallinity in place by setting the material into a mostly amorphous phase 310. The resulting golf ball in state 314 will therefore have a softer cover, because fewer hard crystalline domains 312 remain.
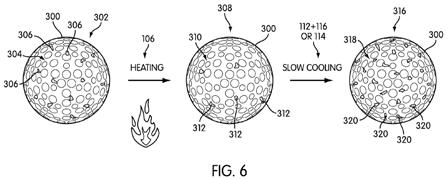
[0047] Conversely, if the end-user desires a harder golf ball cover layer, FIG. 6 shows a representative change in degree of crystallinity as the golf ball undergoes heating step 106 and slow cooling (either steps 112 and 116, or step 114). Golf ball 300 again begins in state 302, with crystalline domains 306 and amorphous phase 304. Again, golf ball 300 undergoes heating step 106, which results in fewer crystalline domains 312 and larger amorphous phase 310. However, in this case, golf ball 300 is allowed to retain heat energy such that the polymer molecules can move and align themselves into crystalline domains during the gradual cooling process. Accordingly, a large number of crystalline domains 320 are formed by the conclusion of the gradual cooling step, and the amorphous phase 318 is accordingly smaller.
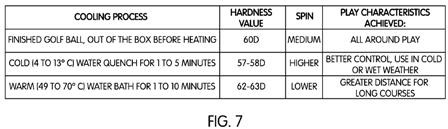
[0048] FIG. 7 shows a sample graphical representation of a correlation between at least one preselected rate of cooling the golf ball and a desired play characteristic exhibited by the golf ball. In the sample graphical representation, the golf ball as manufactured may have a medium hardness value of 60 on the Shore D scale. This medium hardness was discussed above with respect to golf ball 300 in FIGS. 5 and 6 as being initial state 302. The fast cooling step 120 may be described as “cold (4 to 13.degree. C.) water quench for 1 to 5 minutes,” which achieves an lower hardness value of about 57-58 on the Shore D scale. This preselected rate of cooling therefore correlates to higher spin and greater control, which may be useful during cold or wet weather (for example).
[0049] Alternatively, the slow cooling step 112 may be described as “warm (49 to 70.degree. C.) water bath for 1 to 10 minutes.” Slow cooling step 116, whereby the golf ball cools to room temperature, would necessary occur after the golf ball is removed from the warm water bath. This preselected rate of cooling may achieve an increase in hardness, of about 62-63 on the Shore D scale, for example. The resulting golf ball may therefore exhibit lower spin, but increased distance, when hit by a golf club.
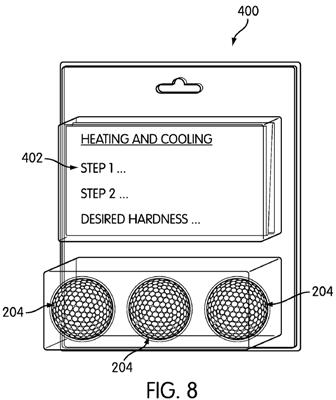
[0050] FIG. 8 shows a sample kit which may be used to carry about the method as discussed variously above. Kit 400 includes at least one golf ball 204, discussed above, and a graphical representation 402 of a correlation between at least one preselected rate of cooling the golf ball (after the golf ball has been heated) and a desired play characteristic exhibited by the golf ball when the golf ball is hit bay a golf club. The graphical representation 402 may also indicate to the end-user
consumer 200 to heat the golf ball to a preselected temperature, and then cool the golf ball at a preselected rate so as to cause the golf ball to exhibit a desired play characteristic.
.
.
.
[0054] Accordingly, the present method and systems of kits allows aftermarket customization of a golf ball. An end-user, or other person, may perform the method on a golf ball so as to achieve a desired play characteristic, and thereby avoid the need to purchase multiple sets of golf balls having different inherent immutable play characteristics. The customization may be repeated multiple times, so as to change the same golf ball from hard to soft (or visa versa) and back again as many times as an end-user may desire. From the perspective of a manufacturer, the method of providing a golf ball and indicating to the user allows the manufacturer to provide a superior system for customizing golf balls to their customers.
Interesting, but sounds like a pain in the neck to me. Is this the future of golf ball technology? Who knows, but it is one of those things that I just have to see to believe.
David Dawsey – Keeping an Eye on Golf Ball Inventions
PS – click HERE to read about a Nike “Device for Heating a Golf Ball”
